Essure Permanent Contraceptive Device

By Sue Claridge
Espoused as being safer and easier, the Essure contraceptive device has left a devastating legacy for many New Zealand women.
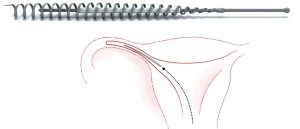
Essure was marketed as a safe, easy alternative to surgical sterilisation; a permanent form of contraception that required no anaesthesia, left no scars. There was no need for a hospital stay. The four-centimetre-long coils could be inserted into a woman’s fallopian tubes in a gynaecologist’s rooms in a short 45 minute procedure, and the woman could go straight back to her day, straight back to her life.
But Essure did not live up to the manufacturer’s hype; not only did the device fail to prevent pregnancy far more often than the alternative tubal ligation, it caused devastating and life-changing harm to thousands of women.
Lynda Williams, AWHC’s co-ordinator for 22 years, wrote in 2013 that Essure was an “insidious experiment [that] has been undertaken on women by obstetrician/ gynaecologists keen to be seen to offering women a new form of permanent contraception. Like many other medical devices, it was released onto the market by the US Food & Drug Administration (FDA) without any decent sized long-term trials or adequate reporting of all the data. So, it was utterly predictable that women began reporting severe problems with the device soon after it came onto the market.”
Over time, so significant were the adverse effects of the device, sales of Essure were suspended in Brazil and the European Union before manufacturer, Bayer, in 2017, withdrew it from sale from all countries around the globe, except the US.[1]
Despite the very poor safety profile, the banning of the device in several countries and the addition of an FDA black box warning in 2016, Bayer insisted that its decision to pull sales and distribution was “not related to a question of safety or dangerousness of the medical device whose positive benefit-risk profile remains unchanged.”[1]
In August 2017, Australasian Medical and Science Ltd (AMSL), in consultation with the Therapeutic Goods Administration, issued a hazard alert for Essure, recalled unused stock and withdrew the device from the Australian market.[2]
In Brazil in February 2017, the regulatory agency Agencia Nacional da Vigilancia Sanitaria (ANVISA) became the first to suspend sales and recall the device, and based their decision on technical and scientific reports.[3] ANVISA categorised the device as presenting maximum risk owing to certain side effects, including:
- changes in menstrual bleeding,
- unwanted pregnancy,
- chronic pain,
- perforation/migration of the device,
- allergy and sensitivity or immune-type reactions.
Following the withdrawal of Essure from every country but the US, The US FDA continued to support use of the device in the US, and in December 2017 said on their website that “the FDA continues to monitor the safety of Essure. The FDA continues to believe that the benefits of the device outweigh its risks, and that Essure’s updated labelling helps to assure that women are appropriately informed of the risks.”[4]
Lawsuits Against Bayer
In early 2018, the Drugwatch website said that Bayer faced thousands of lawsuits in the US as of October 2017 although they had yet to settle in any case.[5]
Drugwatch went on to say that the lawsuits claim Essure complications, including device migration, bleeding, device fracture and other complications requiring surgery, damage to organs and the birth of children with birth defects after the failure of the device to prevent pregnancy. Apparently, Bayer is protected against liability by pre-emption laws, but judges are allowing lawsuits to continue despite this.
In their 2017 Annual Report, Bayer made the following statement:
Essure™: As of January 30, 2018, U.S. lawsuits from approximately 16,100 users of Essure™, a medical device offering permanent birth control with a nonsurgical procedure, had been served upon Bayer. Plaintiffs allege personal injuries from the use of Essure™, including hysterectomy, perforation, pain, bleeding, weight gain, nickel sensitivity, depression and unwanted pregnancy, and seek compensatory and punitive damages. Additional lawsuits are anticipated.
As of January 30, 2018, two Canadian lawsuits relating to Essure™ seeking class action certification had been served upon Bayer. Bayer believes it has meritorious defenses and intends to defend itself vigorously.[6]
In their 2016 Annual Report, Bayer disclosed that they had impairment losses of €391 million (approximately US$413 million) in connection with Essure.[6] In 2017 the Annual report specifically stated[7] that expenses related to significant legal risks “amounted to €258 million in 2017 (2016: €262 million), which, as in the previous year, primarily included expenses in connection with litigation relating to the products Xarelto™, Essure™ and Cipro™/Avelox™.”*
On the 31st of December 2018, Bayer withdrew Essure from the market in the US although it never issued a recall.[8] By 2020 there were almost 39,000 claims against Bayer regarding Essure and in August 2020 “Bayer announced it would pay $1.6 billion to end virtually all of the US Essure lawsuits involving women who claimed the birth control device caused serious health complications.”[8]
Bayer has tried to shift blame for the harm caused by Essure to gynaecologists to absolve itself of liability:
“In the lawsuits that have been allowed to proceed, the manufacturer has used a legal strategy termed the ‘learned intermediary doctrine’ in an effort to shift blame to the gynecologist to absolve itself of liability. The learned intermediary only requires that a manufacturer inform the gynecologist of the risks associated with the device, and the gynecologist, in turn, must notify the patients through adequate informed consent. for Essure injuries mentioned in lawsuits.”[9]
Harm Caused by Essure Devices
A study published in JAMA in 2018 found that women with Essure experienced sterilisation failure seven times more often than women who had their tubes tied,[10] and The Bleeding Edge documentary reported more than 800 pregnancies owing to failure of the Essure devices.[11]
Between October 2013 and October 2023, 62,830 medical device reports about Essure were submitted to the US FDA’s Manufacturer and User Facility Device Experience (MAUDE) database*, of which 118 related to the death of a patient, 1032 were for malfunction of the device, and 61,680 were for injury.[12]
The Medical Device Network reported that between November 2002 and December 2018, most of the injury reports described more than one complication, with 26,244 reports listing pain and 13,114 listing heavier or irregular periods.[13] “Patients also reported headache (8,398), fatigue (6,912), weight fluctuations (5,853), depression/anxiety (5,175), hair loss (4,880) and hypersensitivity/rash (4,807). Many of these symptoms are thought to be caused by autoimmune reactions to the metals Essure is made of.”
In addition, Essure has caused foetal death where the devices have failed and women have become pregnant. The FDA has acknowledged five foetal deaths, but independent analysis says this is a gross underestimate. Madris Kinard (neé Tomes), founder and chief executive officer of Device Events, “said her analysis of thousands of adverse events from the agency’s website shows 303 foetal deaths were linked to Essure.”[14] Ms Kinard is a former FDA Adverse Events Subject Matter Expert for Devices and Unique Device Identification.
Diana Zuckerman, President of the National Center for Health Research, warned that the number of foetal deaths is probably even higher since doctors and patients alike underreport adverse events from Essure.[15]
If the damage that the devices caused, and Bayer’s steadfast refusal to acknowledge that Essure was devastatingly harmful for tens of thousands of women, wasn’t enough, adding insult to injury is the fact that many doctors were paid to millions of dollars to push Essure on to unsuspecting women who would otherwise have opted for other forms of birth control including tubal ligation.
CNN reported in that their “analysis of federal data, shows that from August 2013 through December 2017, Bayer paid 11,850 doctors $2.5 million related to Essure for consulting fees and similar services.”[16]
Dr Martin Makary, a professor of surgery and patient safety expert at Johns Hopkins Medicine, said that although it’s ethical for a pharmaceutical company to pay a doctor for genuine research, he doubted that the more than 11,000 doctors paid by Bayer were involved in such efforts.
“That looks like a bribe,” he said. “That looks like gaming the system. That looks like the pharma company is paying off doctors.”
We have reported on Essure previously in the AWHC Newsletter, most recently in the February-March and April 2018 editions.[17, 18] It has been impossible to obtain any accurate figures on how many women had Essure implanted, although one Auckland gynaecologist, Dr Astrid Budden, wrote in 2014 that she had performed “around 200 procedures” since 2010.[19] When asked in 2018, Bayer would not release how many Essure devices were sold in New Zealand, citing commercial sensitivity.[20] A 2021 Newshub article confirms that it isn’t known how many women in Aotearoa New Zealand received the device.[21]
In our 2018 articles on Essure we wrote that we had been advised that 240 procedures had been done in the Auckland DHB, and 31 in Waitematā. Research by one of the women harmed by Essure in Aotearoa New Zealand suggests that seven DHBs offered the Essure sterilisation.[22] In addition, an unknown number of private practitioners offered the Essure device. Bizarrely, one of those, Omnicare Women’s Health still lists Essure on their website under permanent birth control despite the fact that it was withdrawn from Aotearoa New Zealand in 2017 and Bayer confirmed in 2018 that “no residual stocks remain in the country”![20]
Based on the information we do have, it is conceivable that 1000 or more women/wāhine may have had Essure implanted.
Essure has left a very unwelcome legacy; many women who had the device implanted continue to suffer pain and serious health complications as a result. They struggle to be taken seriously by doctors and often experience gaslighting from health professionals when they seek help. The women we have spoken to for this article have been:
- fobbed off by doctors;
- told their symptoms are nothing to do with Essure;
- told that it’s all in their head;
- accused of being drug-seekers;
- denied the treatment they need to address their symptoms and improve their health and quality of life;
- forced to repeat their story over and over to different doctors, many of whom do not know what Essure is or anything about it, including it’s widely documented adverse impacts and chequered history.
In 2017, when Bayer withdrew the devices from Aotearoa New Zealand, Medsafe issued a recall of Essure on the 8th of September. They stated:
“Unimplanted product is being recalled as the CE† mark has been temporarily suspended. Physicians are being informed of possible adverse events following implantation and being requested to monitor patients.”[23]
There is no evidence that any of the gynaecologists or physicians who implanted the devices have monitored or communicated with affected women at all. There is no mention of Essure on the website of the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG), an organisation that you would expect would have some information for gynaecologists on adverse events associated with these failed devices and Medsafe’s instruction for patients to be monitored. You would expect that it would be encumbent upon the DHBs who implanted Essure to contact each and every patient and discuss their health and potential side-effects of the now thoroughly discredited device.
* * * * *
As well as providing an update on the harm caused by Essure, the aim of this article was to give a voice to some of the women in Aotearoa New Zealand who continue to suffer from the impacts of Essure. Our health entities and our health professionals must be made aware of the impact that their decisions and/or lack of action has on the lives of women who put their faith and trust in the health system, and who have been left to fight that same health system just to regain some semblance of their former lives.
There are, no doubt, many more women in this country suffering in relative silence because of Essure. On behalf of the women we have spoken to and those who continue to suffer, we demand action. The Ministry of Health | Manatū Hauora must issue a genuine “recall” that makes it compulsory for all health professionals and institutional providers involved with the implantation of Essure to contact all women who have had Essure, to provide a thorough check of their wellbeing and health status to establish if they may have suffered from adverse events associated with the device. Urgent follow-up treatment must be offered where required at no cost to the women affected.
In a 2018 article, published in the Sunday Star Times, on the crippling side-effects of Essure, Ministry of Health Medsafe group manager Chris James said that Medsafe expects medical device companies to act responsibly and in patients’ best interests.[24]
“We expect companies to ensure their products are designed using current best practice and that they engage in appropriate post-market surveillance to detect any issues. We also expect them to take action, make changes or issue warnings in good time if they discover problems with their products,” James said.
Such an expectation is tantamount to putting the fox in charge of the hen house. Such mindboggling naivety would be laughable were it not for the devastating and ruinous impact that Essure has had on the lives of so many women in this country. To this day, Bayer insists that Essure is safe and effective.[25]
The problems extend beyond the refusal of some device manufacturers to take responsibility for their faulty and injurious products, to the grossly inadequate regulatory processes for medical devices, especially the FDA’s 510(K) process in the US, upon which Aotearoa New Zealand regulators very largely rely.
We urge all women whose health may have been adversely affected by Essure to lodge complaints with the Health and Disability Commissioner and if further treatment is necessary lodge a claim with ACC through their GP. Additionally, affected women can report their adverse events directly with Medsafe.
(Click here for more information on Reporting Adverse Events and Harm)
* the MAUDE web search feature is limited to adverse event reports within the past 10 years.
† Conformité Européene (CE) Mark, the European Union’s (EU) mandatory conformity marking for regulating the goods sold within the European Economic Area.
References
- Ennis HK, 2017: Bayer Stops Essure Sales Worldwide Except For United States, Ennis & Ennise Attorneys at Law, September 19, 2017.
- Essure contraceptive device: Hazard alert – labelling update relating to potential risks, 30 August 2017, Australian Therapuetic Goods Administration.
- Essure: Suspended in Brazil, Business as Usual in the USA, 3 March 2017, Drugwatch.
- FDA Activities: Essure, accessed in February 2018. (This website has been regularly updated since 2018).
- Michelle Llamas, Essure Lawsuits, Drugwatch, accessed in February 2018.
- Bayer Annual Report 2017 (augmented version), accessed online in February 2018.
- Bayer Annual Report 2016 (augmented version), accessed online in February 2018.
- Drugwatch, 2023: Essure Lawsuits.
- Klimczak AM, 2017: Medicolegal Review: Essure Lawsuits and Legal Strategies Adverse to Gynecologists, Journal of Minimally Invasive Gynecology, 2017 Jul-Aug;24(5):727-730.
- Bouillon K, et al., 2018: Association of Hysteroscopic vs Laparoscopic Sterilization With Procedural, Gynecological, and Medical Outcomes, JAMA. 2018 Jan 23; 319(4): 375–387.
- The Bleeding Edge, Kirby Dick (Director), Netflix, 2018.
- MAUDE – Manufacturer and User Facility Device Experience, US Food and Drug Administration.
- Kent C, 2020: Not very re-Essureing: how a contraceptive implant can ruin lives, Medical Device Network, 6 February, 2020.
- Pierson R, 2016: FDA likely underestimated fetal deaths from Essure: analyst, Healthcare & Pharmaceuticals, Reuters, 18 February 2016.
- Becker: Essure Fetal Deaths May Have Been Misjudged by FDA, Becker Law Office.
- Cohen E and Kessler A, 2018: Bayer paid doctors millions for questionable birth control device, CNN Health, Sat July 28, 2018.
- Claridge S, 2018: Essure – Another Women’s Health Disaster, Auckland Womens Health Council Newsletter, February March 2018.
- Claridge S, 2018: Essure Update, Auckland Womens Health Council Newsletter, April 2018.
- Budden A, 2014: Hysteroscopic sterilisation, Office Gynaecology, Vol. 16 No 3 | Spring 2014.
- Bayer, personal communication, April 2018.
- Kronast H, 2021: Kiwi women reveal their harrowing health battles after receiving permanent contraception Essure, Newshub, 23 October 2021.
- Personal communication, November 2023.
- Medsafe Essure recall notice, Medsafe Online Recall Database.
- Martin H, 2018: Hundreds of Kiwi women had controversial sterilisation performed, Sunday Star Times, 5 August 2018.
- Bayer, 2023: Media Statement – Essure Information, Bayer // Australia/New Zealand, 11 April 2023.
Essure Hysteroscopic Sterilisation

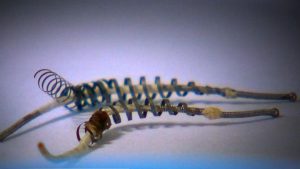
Essure was a permanent sterilisation device that had been offered as a less invasive alternative to tubal ligation, and which was implanted in the fallopian tubes without an incision or anaesthetic. The device comprised an inner core of polyethylene terephthalate (PET) fibres, held in place by a flexible stainless steel inner coil and a dynamic outer nickel titanium alloy coil. The PET fibres were designed to work by stimulating an inflammatory response causing the growth of benign fibrous tissue that blocked the fallopian tube over a period of three months. Women with Essure had to use alternative contraception for three months afterwards, and have hysterosalpingograms at three months to ascertain that the procedure had worked and that the fallopian tubes had become blocked or occluded. (A further hysterosalpingogram would be done at six months if the first showed that the fallopian tubes remained open). The Essure procedure is non-reversible and if side-effects occur the only method of removal is surgery to remove the fallopian tubes. In Aotearoa New Zealand this is only offered as part of a full hysterectomy, although in the US some surgeons offer a limited surgery that removes only the fallopian tubes.

Reports of Essure Harm in Aotearoa New Zealand
In August 2023, AWHC submitted OIA request for information on reports of harm caused by Essure to Medsafe, the Health and Disability Commissioner and ACC. We sought a range of information:
- How many claim/complaints/reports had been lodged regarding the Essure contraceptive device?
- How many claim/complaints/reports were regarding failure of the device to prevent pregnancy?
- How many claim/complaints/reports were regarding pelvic pain, menstrual issues (e.g. heavy bleeding), uterine infection etc?
- How many claim/complaints/reports were regarding systemic issues such as chronic fatigue, headaches/migraine, autoimmune disorders or symptoms, pain, etc?
- How many claim/complaints/reports were regarding sensitivity or allergy issues such as sensitivity to nickel?
- Were any claim/complaints/reports referred to the need for follow-up procedures, such as removal of the Essure devices, surgical removal of fallopian tubes, hysterectomy, etc. as a result of failure or injury caused by the Essure devices?
In addition, we asked ACC how many claims were accepted or declined and how much had been paid out for claims regarding Essure devices. We also asked the HDC the outcomes of the complaints that were lodged.
Medsafe
- Medsafe has received 12 reports of adverse events following implantation of Essure.
- Two reports of pregnancy were received, and in two further cases tubal patency was specified (the devices failed to block the fallopian tubes and pregnancy was possible).
- One of the reports specified heavy menstrual bleeding, and two others referred to pelvic pain. One report referred to chronic fatigue and one to migraine. Three reports referred to pain in addition to the two reports that specified ‘pelvic pain’.
- There were no reports of sensitivity or allergy issues.
- Six of the reports mentioned further surgery including removal.
Health and Disability Commissioner
The HDC response noted that the complaints database does not categorise complaints as to whether or not an Essure device was involved in the complaint. They obtained the data they provided by searching for complaints where the word ‘Essure’ was used in the complaint description field and said that this is unlikely to represent all complaints received by HDC about Essure devices.
Two complaints which mentioned ‘Essure’ in the complaint description field were found.
One complaint resulted in no further action being taken following assessment, and one is currently under assessment by HDC.
Of the two complaints identified in HDC’s database, both complaints involved informed consent issues and concerns over continued pain and unexpected outcomes following implantation of the device. Neither complaint involved a failure to prevent pregnancy. Both complaints involved follow-up procedures and concerns around removal of the device.
ACC
Between 1 July 2005 and 9 September 2023, ACC made a cover decision for nine treatment injury claims that mention the term ‘Essure’. Four claims were accepted for cover and five claims were declined for cover.
To date, the total cost of the four accepted treatment injury claims is $105,547. This amount includes compensation payments of $2,833, rehabilitation payments of $23,704 and treatment payments of $79,010.
The four accepted claims have different primary injuries; foreign body, implants misplaced, perforation and tissue injury/damage.
ACC also noted that until a claim cover decision has been made, they are unable to determine the cause of the injury, in this case, Essure contraceptive device. Therefore, they are unable to provide data for the number of claims lodged relating to Essure.